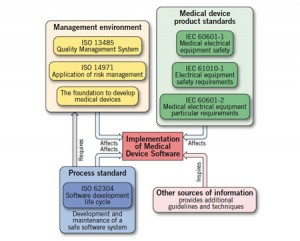
 The FDA approved ISO 62304 as a recognized software development standard in 2009. Developing Medical Device Software to ISO 62304 gives a nice overview.
The FDA approved ISO 62304 as a recognized software development standard in 2009. Developing Medical Device Software to ISO 62304 gives a nice overview.
Besides providing a globally accepted development process one of the other practical components is the assignment of a safety class to individual software items and units:
- Class A: No injury or damage to health is possible
- Class B: Non-serious injury is possible
- Class C: Death or serious injury is possible
Each classification changes the required documentation for the assigned software.
These standards will become more widely known as the FDA moves to regulate the proliferation of medical applications for personal and home use, most notably software that runs on mobile devices. I've discussed this before in When Cell Phones Become Medical Devices. As noted more recently in FDA oversight may extend throughout health IT:
... an FDA director stated flatly: "Under the Federal Food, Drug and Cosmetic Act, HIT software is a medical device."
Broad FDA oversight at the QSR/62304 level will probably not happen, but change is certainly coming for many HIT companies.
The Elsmar Cove Forum IEC 62304 - Medical Device Software Life Cycle Processes has a lot of discussion on this topic. This is where I found a document checklist that is useful for understanding the process scope:
IEC62304_Checklist.xls (Excel spreadsheet)
UPDATE (9/9/10): IEC 62304 – The Basics
Tags: IEC 62304
[…] this article: ISO 62304: The Harmonized Standard for Medical Device Software … Share and […]
[…] This post was mentioned on Twitter by Robert Nadler, 上原 昭宏. 上原 昭宏 said: 医療機器のソフトウェア開発標準規格のお話 RT @bobnadler ISO 62304: The Harmonized Standard for Medical Device Software Development http://bt.io/FMIz […]
Yes – this standard will need to be addressed by medical device product development teams in order to pass FDA inspection and get clearance for devices. It reinforces the need for quality standards and development procedures that address ISO 14791 regarding software risk analysis. Like system hazard analysis which relies on the MDD list of Essential Requirements, formal hazard analysis will need to be conducted for software components, as well.
Hi,
you are using my intellectual property, check the property field of the IEC62304_Checklist.xls file.
Regards
Sz.
The 62304 is a IEC standard and not a ISO standard.
Interesting writing , I am thankful for the facts ! Does someone know where I could possibly locate a template a form version to fill out ?
Hello Travis. my assistant located a fillable CBP 823F copy here http://goo.gl/YX0JXk