 Mobile devices are quickly becoming the conduit of choice for collecting and disseminating clinical data. The FDA will soon be forced to step in and take regulatory control. It’s going to happen eventually.
Mobile devices are quickly becoming the conduit of choice for collecting and disseminating clinical data. The FDA will soon be forced to step in and take regulatory control. It’s going to happen eventually.
Bradley Merrill Thompson does a good job of outlining the factors that lead to FDA oversight in the article FDA may regulate certain mobile phones, accessories. The Components vs. Accessories distinction is an important one for manufacturers — regulatory oversight is dependent on who buys it. The “intended use”, labeling, and marketing are also factors.
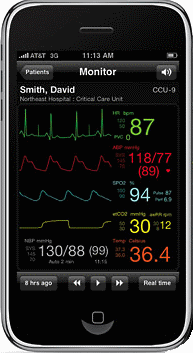
Because of its unique user interface, display, and broadband capabilities the Apple iPhone is a particularly attractive platform for medical applications. For example, the AirStrip OB application is available for download at the Apple App Store and is FDA cleared. Other modalities, like the Critical Care monitor application (shown) is still in testing.
The “intended use” issues are complex. A cell phone that is used to communicate clinical information, e.g. to a PHR, essentially becomes part of a Networked Medical Device.
This mean that 510(k) premarket notification may also be necessary under the proposed Medical Device Data System (MDDS) rules. If you read though what constitutes a MDDS, you can see how well the definitions fit mobile device functionality:
- The electronic transfer or exchange of medical device data from a medical device.
- The electronic storage and retrieval of medical device data.
- The electronic display of medical device data.
- The electronic conversion of medical device data from one format to another format.
Its not the end of the world to be classified as a medical device, but verification and validation of these functions are not a trivial endeavor (see here).
The FDA is almost certainly looking and will be taking action soon.
UPDATE (7/25/09): Here's a mobile device that does not appear to have FDA approval: EKG On Your Mobile Wherever You Are
UPDATE (11/24/09): When will the FDA drop the gavel?
UPDATE (4/19/11): Navigating Regulatory Uncertainty for Smartphones

Pingback: Is FDA Watching Over Cell Phones as Medical Devices? | Medicalcenterinfo.com Blogs
Thank you for the great post. Combined with today’s WSJ article, there is a lot of attention being paid to this topic. (We just covered it and linked to a couple of your posts from Medical Translation Insight.)
Pingback: ISO 62304: The Harmonized Standard for Medical Device Software Development | Bob on Medical Device Software
You’re spot-on that an app on a smartphone that acquires data from a medical device (or wireless physiological sensor) could meet the definition of an MDDS. Now that the final rule is published, this will likely impact some manufacturers.