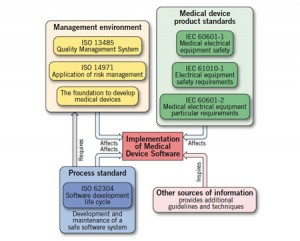
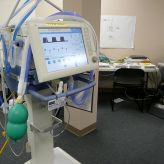
Here's an interesting analysis of security threats within a Windows-based hospital network for embedded medical devices: A threat analysis of critical patient monitoring medical devices.
The threat models are fairly complex and clearly a product of wider enterprise network IT security needs. I've discussed some of the other issues of putting medical devices on an institutional network in Networked Medical Devices. Security threats were not covered and this is an important topic for every hospital network.
There are a couple of items in this article worth commenting on.
The top five unmitigated threats were found to be:

The corrective action for the top threat (T002) was (my highlight):
After it was decided to remove all ePHI from the medical device data storage, the risk assessment changed and the threat of the medical device infecting the hospital enterprise network (T017) then became our primary concern.
This may be the "most effective countermeasure possible for HIPAA compliance and protecting patient privacy", but it is a not practical solution in the real world. Many medical devices store patient demographics. Because the benefits of patient identification outweigh the security risks, this practice is not likely to change in the future.
On these questions:
- Can the medical devices be infected from the enterprise network?
- Can the medical devices be infected via removable media?
- Can infected medical devices propagate malicious software back into the enterprise network?
I generally agree with the conclusions for the device under analysis. The challenge for a hospital is how do you ensure that every networked medical device follows these best practices (communications integrity, hardened OS, clean distribution media, etc.)?
 Tim provides a good starting point for understanding this in EMR Integration for Medical Devices: The Basics.
Tim provides a good starting point for understanding this in EMR Integration for Medical Devices: The Basics.
 The built-in ability to e-mail results to family or a physician seems useful, but posting your blood pressure on Facebook or Twitter? I don't know...
The built-in ability to e-mail results to family or a physician seems useful, but posting your blood pressure on Facebook or Twitter? I don't know...