The MassDevice article Doctors to patients: Track your own health data has an interesting take on the personally controlled healthcare record (PCHR).
The MassDevice article Doctors to patients: Track your own health data has an interesting take on the personally controlled healthcare record (PCHR).
Keely Wray advocates that PCHR data should be "Hackable" (my highlight):
I mean "hackable" in the sense of the word that is quickly growing in popularity: allow owners of a product to manipulate, re-purpose or add to the functionality of a product to serve their own personal needs.
Ms. Wray asks:
Doesn't it make sense to allow patients to put the technologies together in a way that meets their needs?
Their own needs? Maybe yes, but probably not.
The biggest incentive for innovation will be where someone sees an opportunity to meet a lot of other people's needs. This may well be for group that shares a common problem or aliment with the technologist(s). The initial intent may be altruistic, but real growth will only take place when a market emerges. This is the reality that could lead to significant new health data management solutions.
For example, PatientsLikeMe started off this way (from the About Us page):
Our personal experiences with ALS (Lou Gehrig's disease) inspired us to create a community of patients, doctors, and organizations that inspires, informs, and empowers individuals.
There's nothing wrong with that.
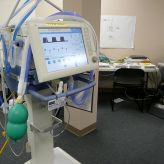 Tim provides a good starting point for understanding this in EMR Integration for Medical Devices: The Basics.
Tim provides a good starting point for understanding this in EMR Integration for Medical Devices: The Basics.
 Jeff Atwood is a great writer. All of his blog posts are informative and interesting.
Jeff Atwood is a great writer. All of his blog posts are informative and interesting.
 The built-in ability to e-mail results to family or a physician seems useful, but posting your blood pressure on Facebook or Twitter? I don't know...
The built-in ability to e-mail results to family or a physician seems useful, but posting your blood pressure on Facebook or Twitter? I don't know...